In Anodonta these pallial tentacles are confined to a small area surrounding
the inferior siphonal notch (fig. 1 [3], t). When the edges
of the mantle ventral to the inhalant orifice are united, an anterior
aperture is left for the protrusion of the foot, and thus there are three
pallial apertures altogether, and species in this condition are called
“Tripora.” This is the usual condition in the Eulamellibranchia
and Septibranchia. When the pedal aperture is small and far
forward there may be a fourth aperture in the region of the fusion
behind the pedal aperture. This occurs in Solen, and such forms are
called “Quadrifora.”
|
|
Fig. 2.—View of the two
Valves of the Shell of
Cytherea (one of the Sinupalliate
Isomya), from the
dorsal aspect.
|
|
|

|
|
Fig. 3.—Right Valve of the same Shell from
the Outer Face.
|
|
The centro-dorsal point a of the animal of Anodonta (fig. 1 [1]) is
called the umbonal area; the great anterior muscular surface h is that
of the anterior adductor muscle, the
posterior similar surface i is that of
the posterior adductor muscle; the long
line of attachment u is the simple
“pallial muscle,”—a thickened ridge
which is seen to run parallel to the
margin of the mantle-skirt in this
Lamellibranch. In siphonate forms the
pallial muscle is not simple, but is indented
posteriorly by a sinus formed by
the muscles which retract the siphons.
It is the approximate equality in the
size of the anterior and posterior adductor
muscles which led to the name
Isomya for the group to which Anodonta
belongs. The hinder adductor muscle
is always large in Lamellibranchs, but
the anterior adductor may be very
small (Heteromya), or absent altogether
(Monomya). The anterior adductor
muscle is in front of the mouth and
alimentary tract altogether, and must
be regarded as a special and peculiar
development of the median anterior part
of the mantle-flap. The posterior adductor
is ventral and anterior to the
anus. The former classification based on these differences in the
adductor muscles is now abandoned, having proved to be an unnatural
one. A single family may include isomyarian, anisomyarian
and monomyarian forms, and the latter in development pass through
stages in which they resemble the first two. In fact all Lamellibranchs
begin with a condition in which there is only one adductor, and that
not the posterior but the anterior. This is called the protomonomyarian
stage. Then the posterior adductor develops, and becomes
equal to the anterior, and finally in some cases the anterior becomes
smaller or disappears. The single adductor muscle of the Monomya
is separated by a difference of fibre into two portions, but neither of
these can be regarded as possibly representing the anterior adductor
of the other Lamellibranchs. One of these portions is more ligamentous
and
serves to keep the
two shells constantly
attached
to one another,
whilst the more
fleshy portion
serves to close the
shell rapidly when
it has been gaping.
In removing the
valves of the shell
from an Anodonta,
it is necessary
not only to cut
through the muscular
attachments
of the body-wall
to the shell but to
sever also a strong
elastic ligament,
or spring resembling
india-rubber, joining the two shells about the umbonal area.
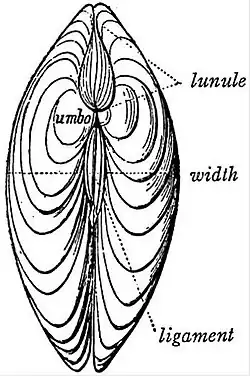
The shell of Anodonta does not present these parts in the most
strongly marked condition, and accordingly our figures (figs. 2, 3, 4)
represent the valves of the sinupalliate genus Cytherea. The corresponding
parts are recognizable in Anodonta. Referring to the figures
(2, 3) for an explanation of terms applicable to the parts of the valve
and the markings on its inner surface—corresponding to the muscular
areas already noted on the surface of the animal’s body—we must
specially note here the position of that denticulated thickening of the
dorsal margin of the valve which is called the hinge (fig. 4). By this
hinge one valve is closely fitted to the other. Below this hinge each
shell becomes concave, above it each shell rises a little to form the
umbo, and it is into this ridge-like upgrowth of each valve that the
elastic ligament or spring is fixed (fig. 4). As shown in the diagram
(fig. 5) representing a transverse section of the two valves of a
Lamellibranch, the two shells form a double lever, of which the
toothed-hinge is the fulcrum. The adductor muscles placed in the
concavity of the shells act upon the long arms of the lever at a
mechanical advantage; their contraction keeps the shells shut, and
stretches the ligament or spring h. On the other hand, the ligament
h acts upon the short arm formed by the umbonal ridge of the shells;
whenever the adductors relax, the elastic substance of the ligament
contracts, and the shells gape. It is on this account that the valves
of a dead Lamellibranch always gape; the elastic ligament is no
longer counteracted by the effort of the adductors. The state of
closure of the valves of the shell is not, therefore, one of rest; when
it is at rest—that is,
when there is no muscular
effort—the valves
of a Lamellibranch are
slightly gaping, and are
closed by the action of
the adductors when the
animal is disturbed. The
ligament is simple in
Anodonta; in many Lamellibranchs
it is separated
into two layers, an outer
and an inner (thicker and
denser). That the condition
of gaping of the
shell-valves is essential
to the life of the Lamellibranch
appears from the
fact that food to nourish
it, water to aerate its
blood, and spermatozoa
to fertilize its eggs, are
all introduced into this
gaping chamber by currents of water, set going by the highly-developed
ctenidia. The current of water enters into the sub-pallial
space at the spot marked e in fig. 1 (1), and, after passing as far forward
as the mouth w in fig. 1 (5), takes an outward course and
leaves the sub-pallial space by the upper notch d. These notches are
known in Anodonta as the afferent and efferent siphonal notches
respectively, and correspond to the long tube-like afferent inferior
and efferent superior “siphons” formed by the mantle in many
other Lamellibranchs (fig. 8).

|
|
Fig. 4.—Left Valve of the same Shell
from the Inner Face. (Figs. 2, 3, 4 from
Owen.)
|
|
|

|
|
Fig. 5.—Diagram
of a section of a
Lamellibranch’s
shells, ligament and
adductor muscle.
a, b, right and left
valves of the shell;
c, d, the umbones or
short arms of the
lever; e, f, the long
arms of the lever;
g, the hinge; h, the
ligament; i, the adductor
muscle.
|
|
Whilst the valves of the shell are equal in Anodonta we find in
many Lamellibranchs (Ostraea, Chama, Corbula, &c.) one valve
larger, and the other smaller and sometimes
flat, whilst the larger shell may be fixed to
rock or to stones (Ostraea, &c.). A further
variation consists in the development of
additional shelly plates upon the dorsal line
between the two large valves (Pholadidae). In
Pholas dactylus we find a pair of umbonal
plates, a dors-umbonal plate and a dorsal
plate. It is to be remembered that the whole
of the cuticular hard product produced on
the dorsal surface and on the mantle-flaps
is to be regarded as the “shell,” of which a
median band-like area, the ligament, usually
remains uncalcified, so as to result in the production
of two valves united by the elastic
ligament. But the shelly substance does not
always in boring forms adhere to this form
after its first growth. In Aspergillum the
whole of the tubular mantle area secretes a
continuous shelly tube, although in the young
condition two valves were present. These
are seen (fig. 7) set in the firm substance of
the adult tubular shell, which has even replaced
the ligament, so that the tube is
complete. In Teredo a similar tube is formed
as the animal elongates (boring in wood),
the original shell-valves not adhering to it
but remaining movable and provided with
a special muscular apparatus in place of a
ligament. In the shell of Lamellibranchs
three distinct layers can be distinguished:
an external chitinous, non-calcified layer, the
periostracum; a middle layer composed of
calcareous prisms perpendicular to the surface,
the prismatic layer; and an internal layer
composed of laminae parallel to the surface,
the nacreous layer. The last is secreted by the
whole surface of the mantle except the border, and additions to its
thickness continue to be made through life. The periostracum is
produced by the extreme edge of the mantle border, the prismatic
layer by the part of the border within the edge. These two layers,
therefore, when once formed cannot increase in thickness; as the
mantle grows in extent its border passes beyond the formed parts
of the two outer layers, and the latter are covered internally by a
deposit of nacreous matter. Special deposits of the nacreous matter
around foreign bodies form pearls, the foreign nucleus being usually
of parasitic origin (see Pearl).



