Life Movements in Plants Vol 1/Chapter 12
XII.—THE EFFECT OF CHEMICAL AGENTS ON GROWTH
By
Sir J. C. Bose,
Assisted by
Guruprasanna Das.
Chemical agents are found to exert characteristic actions on growth. The method of investigation sketched here opens out an extended field of investigation. The effect of a chemical substance, I find, to be modified by (1) the strength of the solution, (2) the duration of application, and (3) the condition of the tissue. A poisonous substance in minute doses is often found to exert a stimulating action. Too long continued action of a stimulant, on the other hand, exerts a depressing effect. The influence of the tonic condition is shown by the fact that while a given dilution of a poisonous substance kills a weak specimen, the same poisonous solution, applied to a vigorous specimen, actually stimulates and enhances the rate of the growth. I give below descriptions of a few typical reactions.
The reagent, when in a liquid form, is locally applied on the growing organ. The records, taken before and after the application, exhibit the stimulatory or depressing character of the reagent. A different method of application of the reagent is employed for plants with extended region of growth. The specimen is then enclosed in a glass cylinder, with inlet and outlet pipes. The cylinder is first filled with water, and the normal rate of growth recorded. This rate remains constant for several hours; but prevention of access of air for too long a time affects the normal growth. After obtaining normal record, water charged with the giving chemical agent is passed into the cylinder; and the subsequent record shows the characteristic effect of the reagent. The introduction of a gas into the chamber offers no difficulty.
EFFECT OF STIMULANTS.
Hydrogen Peroxide: Experiment 62.—This reagent, as supplied by Messrs. Parke Davis & Co., was diluted to 1 per cent. and applied to the growing plant. Its stimulating action on growth is demonstrated in the right hand record of Fig. 68a, where the rate of growth is soon enhanced two and a half times the normal rate.
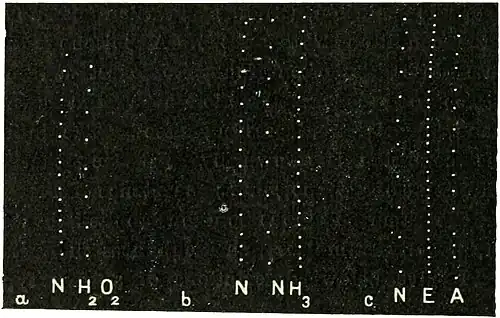
Fig. 68.—Effect of chemical agents: (a) Acceleration of growth under H2O2, (b) Effect of NH3, preliminary acceleration followed by retardation. (c) Effect of ether (E) and recovery (A).
Ammonia: Experiment 63.—The immediate effect of dilute vapour of this reagent is an enhancement of growth, seen in the middle record of Fig. 68b, where the rate is seen to be double the normal. Continued action, however, caused a depression; the third record of this series shows this, where the reduction is three-fourths of the normal rate.
EFFECT OF ANÆSTHETICS.
Ether: Experiment 64.—In Fig. 68c, the records exhibit the effect of introduction of ether vapour into the plant chamber, and its recovery after the removal of the vapour. Ether is seen to depress the rate of growth to a little more than a third of the normal rate. The recovery is seen to be nearly complete half an hour after the removal of the vapour.
Carbonic Acid: Experiment 65.—The action of this gas is very remarkable. The plant was immersed in water and normal record taken;
Fig. 69.—Effect of CO2. (a) Normal record; (b) immediately after application of CO2 and (c) 15 minutes after. the plant chamber was now filled with water, charged with carbonic acid gas. This induced a very marked acceleration of growth (Fig. 69). In a seedling of Onion, the increase was found to be two and a half times. In the flower bud of Crinum, the rate was found enhanced threefold from the normal 0.25 μ to 0.75 μ per second. After this preliminary enhancement, there was a depression of growth within 15 minutes of the application, the rate being new reduced to 0.15 μ per second. These effects were found to take place equally in light or in darkness.
ACTION OF DIFFERENT GASES.
Coal Gas: Experiment 66.—Coal gas induces a depression. It is curious that subjection to the action of this gas does not produce so evil an effect as one would expect. The introduction of the gas had reduced the growth-rate to more than half; but there was a recovery half an hour after the introduction of fresh air.
Sulphuretted Hydrogen: Experiment 67.—This gas not only exerts a depressing effect, but its after-effect is also very persistent. The plant experimented on was very vigorous and its rate of growth was depressed to half by subjection to the action of the gas for a short time. The record taken half an hour after the introduction of fresh air did not exhibit any recovery.
ACTION OF POISONS.
Ammonium Sulphide: Experiment 68.—This reagent in dilute solution retards growth, and in stronger solution acts as a poison. The following results were obtained with a wheat seedling under different strengths of solution:—
| Normal rate | … | 0.30 μ per sec. |
| 0.5 per cent. solution | … | 0.15 μ per„ sec.„ |
| 2.0 per„ cent.„ solution„ | … | 0.08 μ per„ sec.„ |
Copper Sulphate: Experiment 69.—The effect of a solution of this reagent is far more depressing than the last. One per cent. solution acting for a short time depressed the rate from 0.45 μ to 0.13 μ per sec. Long continued action of the poisonous solution kills the plant.
SUMMARY.
The effect of a chemical agent is modified by the strength of the solution, the duration of application and the tonic condition of the tissue.
Dilute solution of hydrogen peroxide induces an acceleration of growth.
The action of dilute vapour of ammonia is a preliminary enhancement followed by depression of growth.
Ether vapour depresses the rate of growth. On the removal of the vapour there is a recovery of the normal rate.
The effect of carbonic acid is a great enhancement of the rate of growth; after this preliminary action, growth undergoes a decline. The effect described takes place equally in light or in darkness.
Coal gas induces a depression of the rate of growth from which there is a recovery after the removal of the gas. The action of sulphuretted hydrogen is far more toxic, the after-effect being very persistent.
Solution of ammonium sulphide induces increasing retardation of growth, with the strength of the solution. Copper sulphate solution acts as a toxic agent, retarding the rate of growth and ultimately killing the plant.