1911 Encyclopædia Britannica/Pyridine
PYRIDINE, C5H5N, an organic base, discovered by T. Anderson (Trans. Roy. Soc. Edin., 1851, 20, p. 251) in bone oil. It is also found among the distillation products of bituminous coal, lignite, and various shales, and has been detected in fusel oil and crude petroleum. It is a decomposition product of various alkaloids (nicotine, sparteine, cinchonine, &c.), being formed when they are strongly heated either alone or with zinc dust. It may be synthetically prepared by distilling allyl ethylamine over heated lead oxide (W. Königs, Ber., 1879, 12, p. 2341) by passing a mixture of acetylene and hydrocyanic acid through a red-hot tube (W. Ramsay, Ber., 1877, 10, p. 736); by heating pyrrol with sodium methylate and methylene iodide to 200° C. (M. Dennstedt and J. Zimmermann, Ber., 1885, 18, p. 3316), by heating isoamyl nitrate with phosphorus pentoxide (E. T. Chapman and M. H. Smith, Ann., 1868, Suppl. 6, p. 329); and by heating piperidine in acetic acid solution with silver acetate (J. Tafel, Ber., 1892, 25, p. 1619). The amount of pyridine produced in most of these processes is very small, and the best source for its preparation is the “light-oil” fraction of the coal-tar distillate. The basic constituents are removed by dilute sulphuric acid, the acid layer removed, and the bases liberated by alkali, separated, dried, and fractionally distilled.
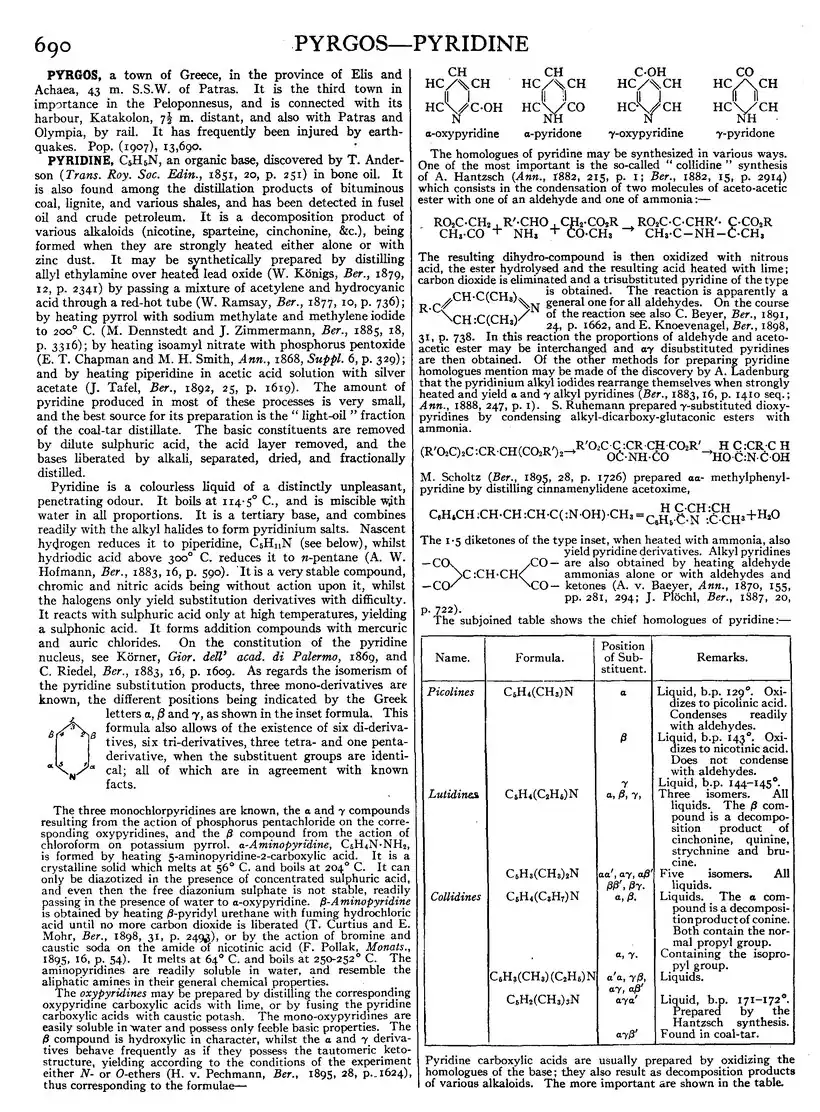
Pyridine is a colourless liquid of a distinctly unpleasant, penetrating odour. It boils at 114·5° C., and is miscible with water in all proportions. It is a tertiary base, and combines readily with the alkyl halides to form pyridinium salts. Nascent hydrogen reduces it to piperidine, C5H11N (see below), whilst hydriodic acid above 300° C. reduces it to n-pentane (A. W. Hofmann, Ber., 1883, 16, p. 590). It is a very stable compound, chromic and nitric acids being without action upon it, whilst the halogens only yield substitution derivatives with difficulty. It reacts with sulphuric acid only at high temperatures, yielding a sulphonic acid. It forms addition compounds with mercuric and auric chlorides. On the constitution of the pyridine nucleus, see Körner, Gior. dell’ acad. di Palermo, 1869, and C. Riedel, Ber., 1883, 16, p. 1609. As regards the isomerism of the pyridine substitution products, three mono-derivatives are known, the different positions being indicated by the Greek letters α, β and γ, as shown in the inset formula. This formula also allows of the existence of six di-derivatives, six tri-derivatives, three tetra- and one penta-derivative, when the substituent groups are identical; all of which are in agreement with known facts.
The three monochlorpyridines are known, the α and γ compounds resulting from the action of phosphorus pentachloride on the corresponding oxypyridines, and the β compound from the action of chloroform on potassium pyrrol. α-Aminopyridine, C5H4N·NH2, is formed by heating 5-amino pyridine-2-carboxylic acid. It is a crystalline solid which melts at 56° C. and boils at 204° C. It can only be diazotized in the presence of concentrated sulphuric acid, and even then the free diazonium sulphate is not stable, readily passing in the presence of water to α-oxypyridine. β-Aminopyridine is obtained by heating β-pyridyl urethane with fuming hydrochloric acid until no more carbon dioxide is liberated (T. Curtius and E. Mohr, Ber., 1898, 31, p. 2493), or by the action of bromine and caustic soda on the amide of nicotinic acid (F. Pollak, Monats., 1895, 16, p. 54). It melts at 64° C. and boils at 250–252° C. The aminopyridines are readily soluble in water, and resemble the aliphatic amines in their general chemical properties.
The oxypyridines may be prepared by distilling the corresponding oxypyridine carboxylic acids with lime, or by fusing the pyridine carboxylic acids with caustic potash. The mono-oxypyridmes are easily soluble in water and possess only feeble basic properties. The 5 compound is hydroxylic in character, whilst the α and δ derivatives behave frequently as if they possess the tautomeric ketostructure, yielding according to the conditions of the experiment either N- or O-ethers (H. v. Pechmann, Ber., 1895, 28, p.,1624), thus corresponding to the formulae—
The homologues of pyridine may be synthesized in various ways. One of the most important is the so-called “collidine” synthesis of A. Hantzsch (Ann., 1882, 215, p. 1; Ber., 1882, 15, p. 2914) which consists in the condensation of two molecules of aceto-acetic ester with one of an aldehyde and one of ammonia:—
RO2C·CH2
CH3·CO +R′·CHO
NH3 + CH2·CO2R
ĊO·CH3 ⟶ RO2C·C·CHR′· C·CO2R
CH3·C—NH—Ċ·CH3
The resulting dihydro-compound is then oxidized with nitrous acid, the ester hydrolysed and the resulting acid heated with lime; carbon dioxide is eliminated and a trisubstituted pyridine of the type
| R·C |
CH·C(CH3) | |
| CH:C(CH3) |
is obtained. The reaction is apparently a general one for all aldehydes. On the course of the reaction see also C. Beyer, Ber., 1891, 24, p. 1662, and E. Knoevenagel, Ber., 1898, 31, p. 738. In this reaction the proportions of aldehyde and aceto-acetic ester may be interchanged and αγ disubstituted pyridines are then obtained. Of the other methods for preparing pyridine homologues mention may be made of the discovery by A. Ladenburg that the pyridinium alkyl iodides rearrange themselves when strongly heated and yield α and γ alkyl pyridines (Ber., 1883, 16, p. 1410 seq.; Ann., 1888, 247, p. 1). S. Ruhemann prepared γ-substituted dioxypyridines by condensing alkyl-dicarboxy-glutaconic esters with ammonia.
R′O2C:CR·CH(CO2R′)2⟶
R′O2C·C:CR·CH·CO2R′
OĊ·NH·ĊO⟶ H C:CR·C H
HO·C̈:N·Ċ·OH
M. Scholtz (Ber., 1895, 28, p. 1726) prepared αα- methylphenylpyridine by distilling cinnamenylidene acetoxime,
C6H5CH:CH·CH:CH·C(:N·OH)·CH3=H C·CH:CH
C6H5·C̈·N :Ċ·CH3+H2O
| —CO | CO— | ||
| —CO | CO— |
The 1·5 diketones of the type inset, when heated with ammonia, also yield pyridine derivatives. Alkyl pyridines are also obtained by heating aldehyde ammonias alone or with aldehydes and ketones (A. v. Baeyer, Ann., 1870, 155, pp. 281, 294; J. Pochl, Ber., 1887, 20, p. 722).
The subjoined table shows the chief homologues of pyridine:—
| Name. | Formula. | Position of Sub- stituent. |
Remarks. |
| Picolines | C5H4(CH3)N | α | Liquid, b.p. 129°. Oxidizes to picolinic acid. Condenses readily with aldehydes.
|
| β | Liquid, b.p. 143°. Oxidizes to nicotinic acid. Does not condense with aldehydes.
| ||
| γ | Liquid, b.p. 144–145°. | ||
| Lutidines | C5H4(C2H5)N | α, β, γ, | Three isomers. All liquids. The β compound is a decomposition product of cinchonine, quinine, strychnine and brucine.
|
| C5H3(CH3)2N | αα′ αγ, αβ′ | Five isomers. All liquids. | |
| Collidines | C5H4(C3H7)N | α, β. | Liquids. The α compound is a decomposition product of conine. Both contain the normal propyl group.
|
| α, γ. | Containing the isopropyl group.
| ||
| C5H3(CH3)(C2H5)N | α′α, γβ αγ, αβ′ |
Liquids. | |
| C5H2(CH3)3N | αγα′ | Liquid, b. p. 171–172°. Prepared by the Hantzsch synthesis.
| |
| αγβ′ | Found in coal-tar. |
Pyridine carboxylic acids are usually prepared by oxidizing the homologues of the base; they also result as decomposition products of various alkaloids. The more important are shown in the table.
| Name. | Formula. | Position of Sub- stituent. |
Remarks. |
| Picolinic acid. |
C5H4(CO2H)N | α | M.p. 137°. Easily soluble in water. Yellow coloration with FeSO4. Position of carboxyl group determined by synthesis from α-naphthylamine (Z. Skraup and A. Cobenzl, Monats., 1883, 4. p. 436).
|
| Nicotinic acid. |
C5H4(CO2H)N | β | M.p. 228–229°. An oxidation product of nicotine, hydrastine and berberine. Constitution determined by synthesis from β-naphthylamine (Skraup).
|
| Quinolinic acid. |
C5H3(CO2H)2N | αβ | M.p. 192–195° with decomposition into nicotinic acid. Formed by oxidation of quinoline.
|
| Cinchomeronic acid. |
C5H3(CO2H)2N | βγ | M.p. 258–259°. Formed by oxidation of quinoine, cinchonine, and isoquinoline.
|
| α-Carbochinco- meronic acid. |
C5H2(CO2H)3N | αβγ | M.p. 249–250°. Crystallizes with 1+1/2H2O. An oxidation product of cinchonine, quinine and papaverine. |
| Berberonic acid. |
C5H2(CO2H), | βγ | M.p. 243°. An oxidation product of berberine. Gives a red coloration with FeSO4. Boiling with glacial acetic acid gives cinchomeronic acid.
|
Trigonelline, C7H7NO2, the methyl betaine of nicotinic acid, was discovered in 1885 by E. Jahns (Ber., 1885, 18, p. 2518), and is found in the seeds of Trigonella and Strophanthus hispidus. It is very soluble in water. With baryta it yields methylamine, and when heated with concentrated hydrochloric acid, to 260° C. it yields methyl chloride and nicotinic acid. It was synthesized by A. Hantzsch (Ber., 1886, 19, p. 631) by condensing methyl iodide and potassium nicotinate at 150° C. the resulting iodide being then decomposed by moist silver oxide. A. Pictet (Ber., 1897, 30, p. 2117) obtained it by oxidizing nicotine methyl hydroxide with potassium permanganate. Apophyllenic acid, C8H7NO4-HZO, the methyl betaine of cinchomeronic acid, was synthesized by W. Roser (Ann., 1886, 234, p. 118).
Piperidine or hexa-hydro pyridine, C5H11N, was first obtained in 1848 by distilling piperine with lime. It is formed in the hydrolysis of piperine by alcoholic potash, by the reduction of trimethylene cyanide (A. Ladenburg) and by the action of alkalis on ε-chloramylamine, Cl(CH2)5-NH2 (S. Gabriel, Ber., 1892, 25, p. 421). It is also produced in the electrolytic oxidation of N-nitroso piperidine in sulphuric acid solution (F. B. Ahrens, Ber., 1898, 31, p. 2275). It is a liquid which boils at 105-106° C., and possesses an ammonia cal smell. It is readily soluble in water, alcohol and ether, and is a very powerful base. It is oxidized to pyridine by heating with concentrated sulphuric acid to 300° C., or with nitrobenzene to 250° C., or with silver acetate to 180° C. Being an imide it readily yields a nitroso derivative, and N-alkyl and acidyl derivatives. The piperidine ring is easily split. When heated with fuming hydriodic acid to 300° C. it yields normal pentane and ammonia, and hydrogen peroxide oxidizes it to glutarimide and to a piperidinium oxide or oxime (R. Wolffenstein, Ber., 1904, 37, p. 3228). A. W. Hofmann (Ber. 1881, 14, p. 660), by a process of exhaustive methylation and distillation, obtained the unsaturated hydrocarbon piperylene, CH2:CH·CH2·CH:CH2 from piperidine (see also A. Ladenburg, Ann., 1894, 279, p. 344)
- C5H11N(+CH3I)⟶C5H10N(CH3)2I(+AgOH)⟶ C5H10N(CH3)2·OH
- (distil)↓
- C5H9N(CH3)3·OH⟵('+AgOH)C5H9N(CH3)3I⟵(+CH3l)C5H9N(CH3)2
- (distil)↓
- C5+N(CH3)3+H2O
J. v. Braun (Ber., 1904; 37, p. 2915) showed that benzoyl pieridine, when heated with phosphorus pentachloride to 200° in sealed tubes, yields benzonitrile, and pentamethylene dichloride, thus leading to a simple method of preparing pentamethylene compounds. At 125-130° C. the compound C6H5C-Cl:N(CH¢)5-Cl is obtained; this with water yields benzoylamidochloramylamine C5H5CONH(CH2)5Cl, which when heated with hydrochloric acid tc 170-180° C. furnishes e-chloramylamine, NH¢(CH2)5Cl. a-Propy1piperidine is the alkaloid conine (q.v.).